Abstract
Background: Several biological markers have been reported to predict response to treatment and mortality in patients with acute graft-versus-host disease (aGVHD) after allogeneic hematopoietic stem cell transplant. aGVHD is a major concern since it significantly impacts outcome, especially in patients with steroid refractory aGVHD, leading to a high mortality rate (>50%). The present study was designed to validate a prognostic score based on 6 plasmatic biomarkers: Elafin, IL2Ra, TNFRI, HGF, IL8, and REG3a (Levine et al. Blood 2012).
Methods: A prospective study was conducted between 2013 and 2017 in our institution in patients presenting with aGVHD after transplantation for hematological disease. One blood sample was taken at diagnosis of aGVHD and clinical data were prospectively collected. No response at day 30 after aGVHD onset was defined as non-complete response or requirement of a second line therapy. Association of biomarkers with day-30 response and overall survival was analyzed by logistic and Cox models, respectively. Discriminative ability of the models was quantified by the c index. The biomarkers were measured by a multiplex Luminex based assay (R&D systems) except one which was measured by ELISA (REG3a).
Patient characteristics: Median age at inclusion was 45 years, 63 (36 %) patients were women; hematological disease prior transplant was acute myeloid leukemia or myelodysplastic syndrome in 36 (20%), lymphoma in 31 (18%), acute lymphoid leukemia in 32 (18%) and myeloproliferative neoplasms in 24 (14%) patients. A reduced intensity conditioning regimen was used in 117 (66%) and the donor was an HLA-matched sibling donor in 51 (29%) patients, matched unrelated in 82 (46%) and HLA-mismatched in 44 (25%). Sources of stem cells were G-CSF mobilized peripheral blood stem cells in 138 (78%), bone marrow in 23 (13%) and unrelated cord blood in 16 (9%) patients. aGVHD occurred at a median of 22 days after transplant (range 6 - 77) and was graded I, II and III at time of aGVHD diagnosis for 12%, 57% and 31% of patients respectively. At inclusion, aGVHD involved skin (n=143), gut (n=138) and/or liver (n=29). At day 30 after treatment initiation, 123 (69%) patients had a complete response. The follow-up was greater than 6 months at time of analysis for all living patients. Forty patients were dead 180 days after the aGVHD episode.
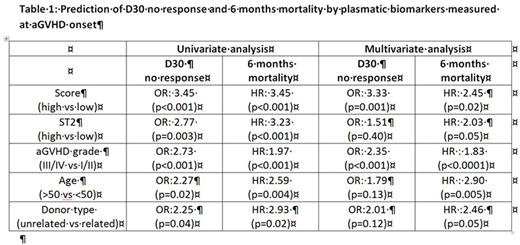
Results: Plasmatic biomarkers were measured at time of corticosteroids initiation at aGVHD onset in 177 patients. Predictive potential was assessed on response at day 30 and mortality at 6 months. The score was significantly associated with day-30 response and 6-month mortality, as already reported. In a multivariable analysis, after adjustment for aGVHD grade at initiation of treatment, age, donor type (related or unrelated), source of stem cells, albumin level and fever, the score was still predictive for day-30 response and 6-month mortality, along with aGVHD grade (table 1). None of the other parameters were associated with day-30 response while age and donor type were associated with 6-months mortality. The same multivariate analysis was further conducted with ST2, another strong prognostic biomarker previously described (Vander Lugt et al NEJM 2013). After adjustment for the other variables, ST2 was associated with 6-month mortality only (table 1).
Finally, we used the c-index to estimate the discriminative ability of the score alone, ST2 alone or the combination of score and ST2. The score had the highest value to discriminate response at day-30 (c-index=0.68) as compared to ST2 (c-index=0.60) and the combination score+ST2 (c-index=0.66). The combination score+ST2 had the highest value to predict 6-month mortality (c-index=0.68) as compared to the score (c-index=0.64) and ST2 (c-index=0.59) alone.
Conclusion: The present study shows the potential of a score based on 6 biomarkers (Elafin, IL2Ra, TNFRI, HGF, IL8, and REG3a) and of ST2, to predict severity of aGVHD, after adjustment for other variables. However, when tested for discriminative value by the c-index, our results support the need for improved biomarker studies and prospective trials designed to re-enforce immunosuppressive therapy in patients with unfavorable biomarkers, while patients with favorable biomarkers could possibly benefit from less intensive aGVHD therapy. This study corroborates the ability of a score based on 6 biomarkers to predict severity of aGVHD in an independent cohort of patients.
Peffault De Latour: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding. Socié: Alexion Pharmaceuticals, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal